Research
- Development of nano-scale wiredrawing: Molecular Dynamics Analysis of defect behavior in iron and steel wire
- Atomistic Study on the Performance of Electrolytes in Lithium Ion Batteries(LIB): Evaluation of Li+ diffusion, viscosity and ionic conductivity
- Plastic Deformability and Strength Evaluation of Silicon Based Hard Brittle Material (SiC)
- Mechanical Transmission in Hierarchical Structure of Biological Fibrillar Materials (Collagen / Cellulose Nano-sized Fibers): Twisting Force and Its Energy Transmission from Micro to Macro
- Multi-scale Modeling and Analysis of Solid Materials: Collaboration between Molecular Dynamics and Macroscopic Particle Method (Peridynamics Theory)
- Nano-scale Tribology and Plastic Deformation: The Effect of Nano-sized texturing on Friction
- Computer Simulation Methodology for Dynamic behavior of Solid Materials
- Universality of Structures based on Mechanical Function: Development from Molecular Structures to the Concept of Tensegrity
Atomistic Study on the Performance of Electrolytes in Lithium Ion Batteries(LIB): Evaluation of Li+ diffusion, viscosity and ionic conductivity
Among chemical ones, batteries that can be repeatedly charged and discharged are called "secondary batteries". Currently, secondary batteries are mainly marketed with lead storage batteries, nickel-metal hydride batteries, and lithium ion batteries. In recent years, however, the demand for lithium-ion batteries (LIB) is rapidly expanding with the miniaturization and weight saving of equipment. Due to the spread of mobile terminals such as mobile phones, smart phones, notebook computers, and owing to the advant of electric vehicles, secondary batteries are required to be compact, lightweight, large capacity, andrapidly rechargeable. A LIB is recognized as a secondary battery that can satisfy these requirements.
A LIB has higher energy density than other secondary batteries, so that it is suitable for miniaturization and weight reduction. Besides, it has a feature that its electric output with respect to weight is very large. However, while it has a large capacity and high output, it has safety problems such as ignition accidents. In addition to further improvement in the performance of LIB, a solution method for safety problems is currently being sought.
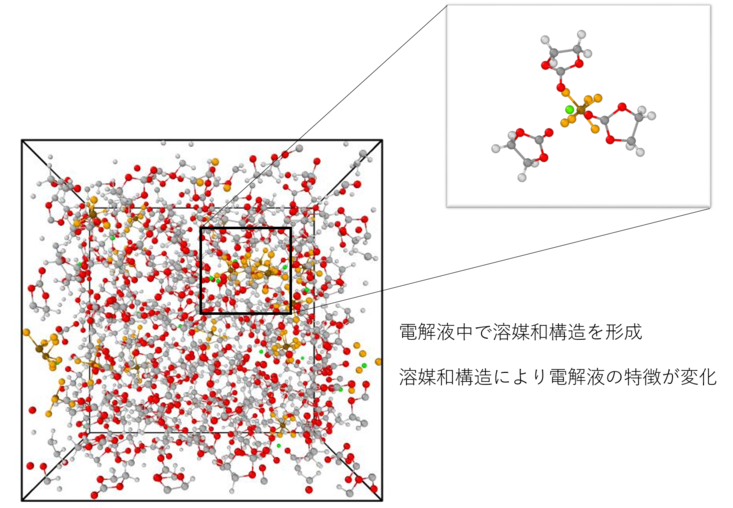
Recent research focuses on the behavior of lithium ions in electrolyte, especially lithium ion behavior is very important because it directly affects battery performance. In this laboratory, molecular dynamics (MD) method is used to analyze microscopic behavior of lithium ions in electrolyte and solvation structure, and is utilized to evaluate physical properties of new compound,such as diffusion coefficient, viscosity and ion conductivity, needed for development of new electrolyte.